Research
Our computational molecular science toolkit:

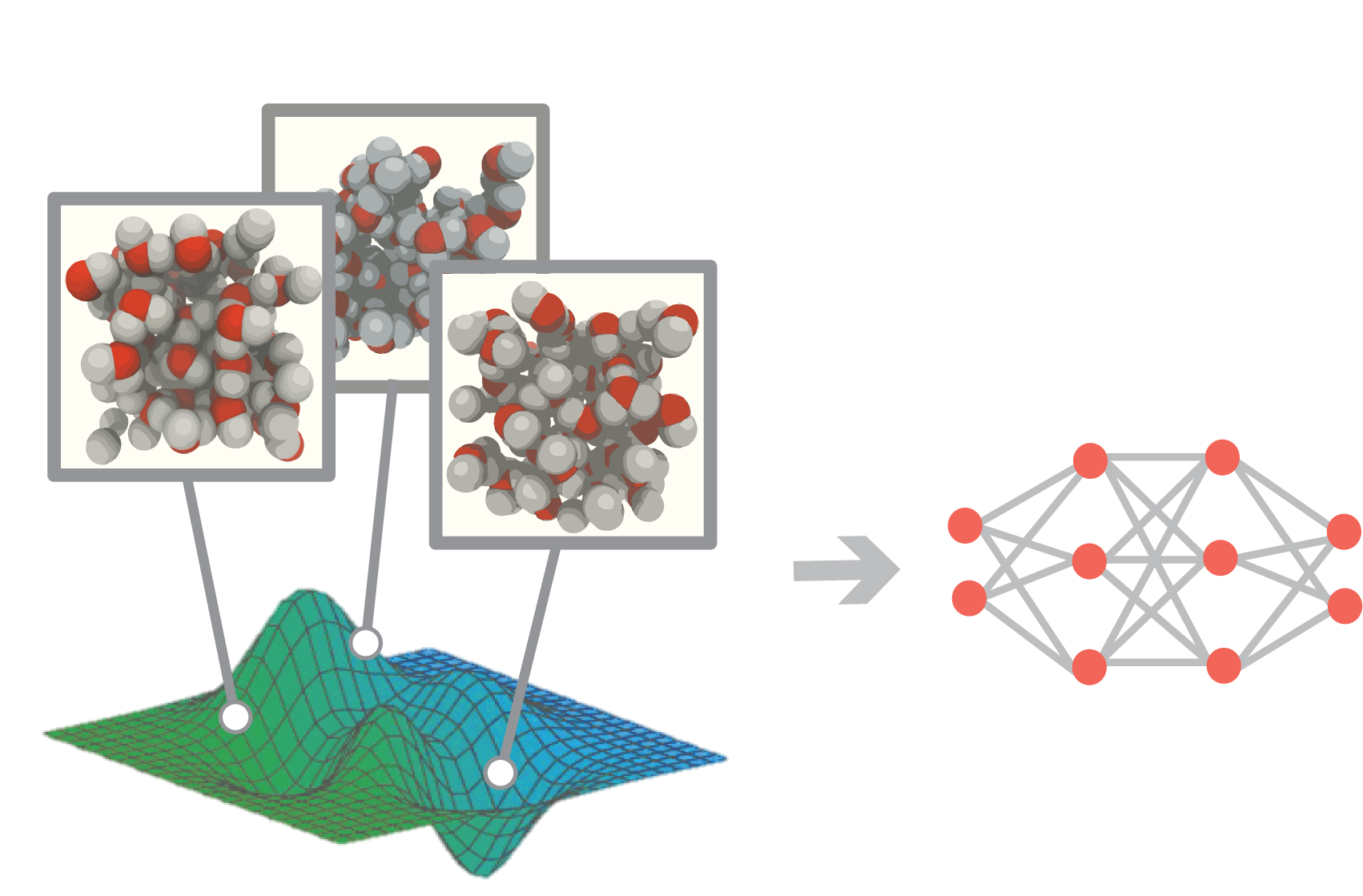
Physics-inspired ML potentials
Molecular simulation is a powerful tool for predicting properties of materials and fluids, but the reliability of these predictions heavily depends on the models used. The challenge in developing these models is a trade-off between the degree of chemical detail (level of theory used) and their computational cost (how fast they can be evaluated on supercomputers). We leverage physics-inspired machine-learning frameworks to build models that avoid this trade-off to predict large-scale thermodynamic properties at first-principles levels of accuracy.
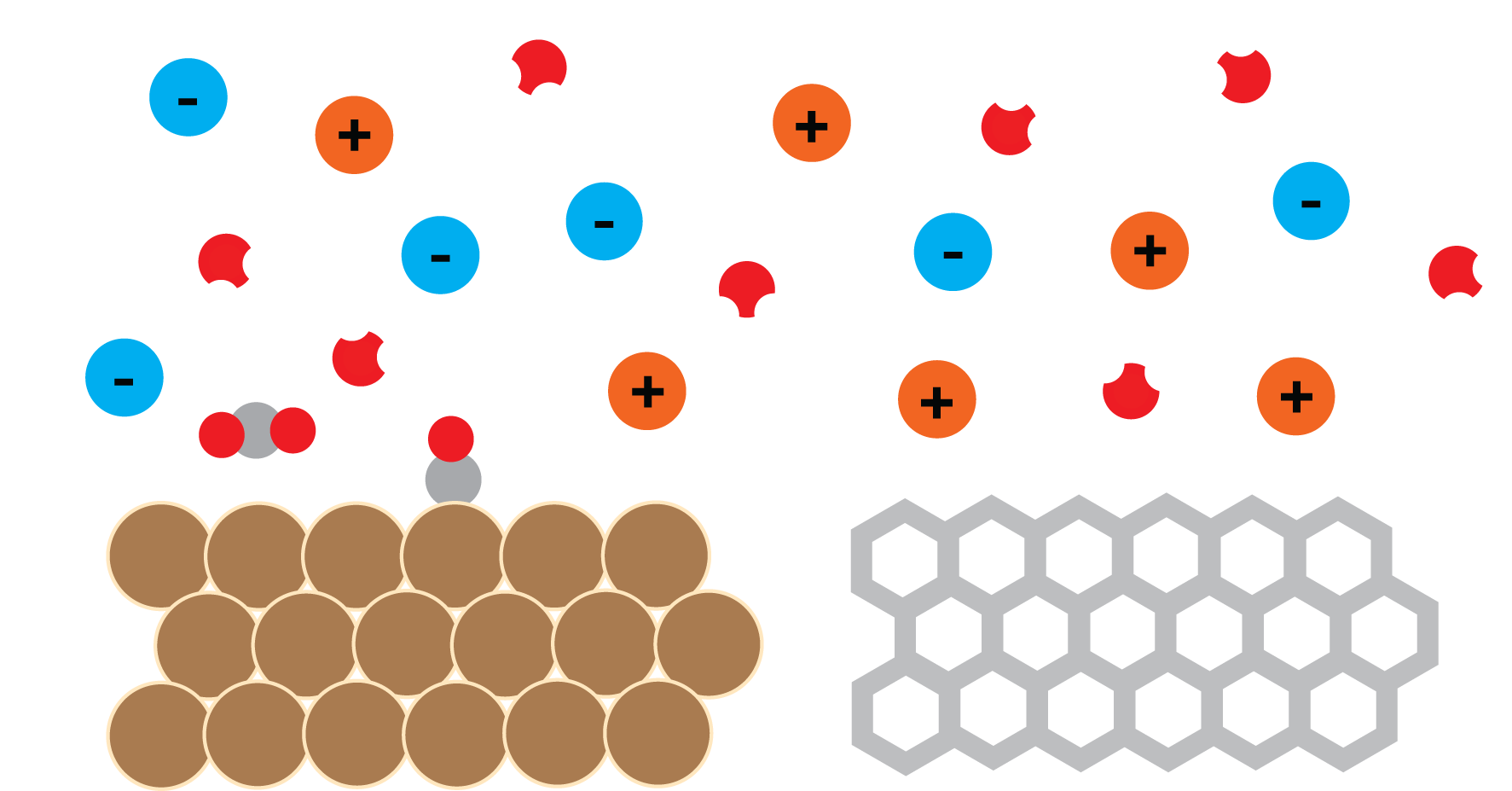
Fluids at interfaces and in confinement
How molecules behave at an interface or in confinement is vastly different from how they behave in bulk. These types of environments are where analytical theories tend to break down, where timescale trends diverge, and where chemical reactions are more likely to happen. Our group seeks to decipher the intricate relationships between surface features, geometries, and the subsequent influence on fluid behavior. We aim to design unique surfaces and electrolyte chemistry targetting optimal separations and catalysis processes.
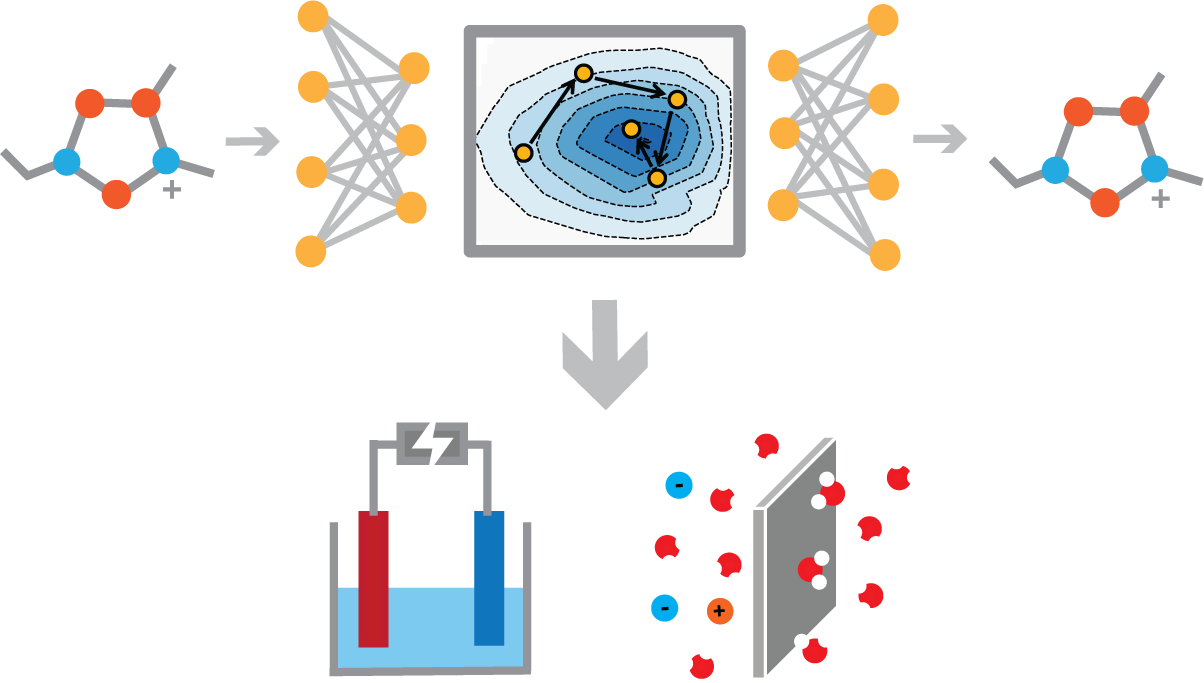
Generative design for ion transport
A key goal in designing energy storage solutions is to identify solvents which enhance charge transport and stability. We leverage machine-learning tools to map molecular structures to transport properties and optimize for desired properties in lower dimensional spaces. This approach allows us to greatly accelerate the exploration and discovery of new electrolytes chemistry that can unlock ion transport behavior surpassing conventional limitations.
